New PPI launched
In Clinical
Follow this topic
Bookmark
Record learning outcomes
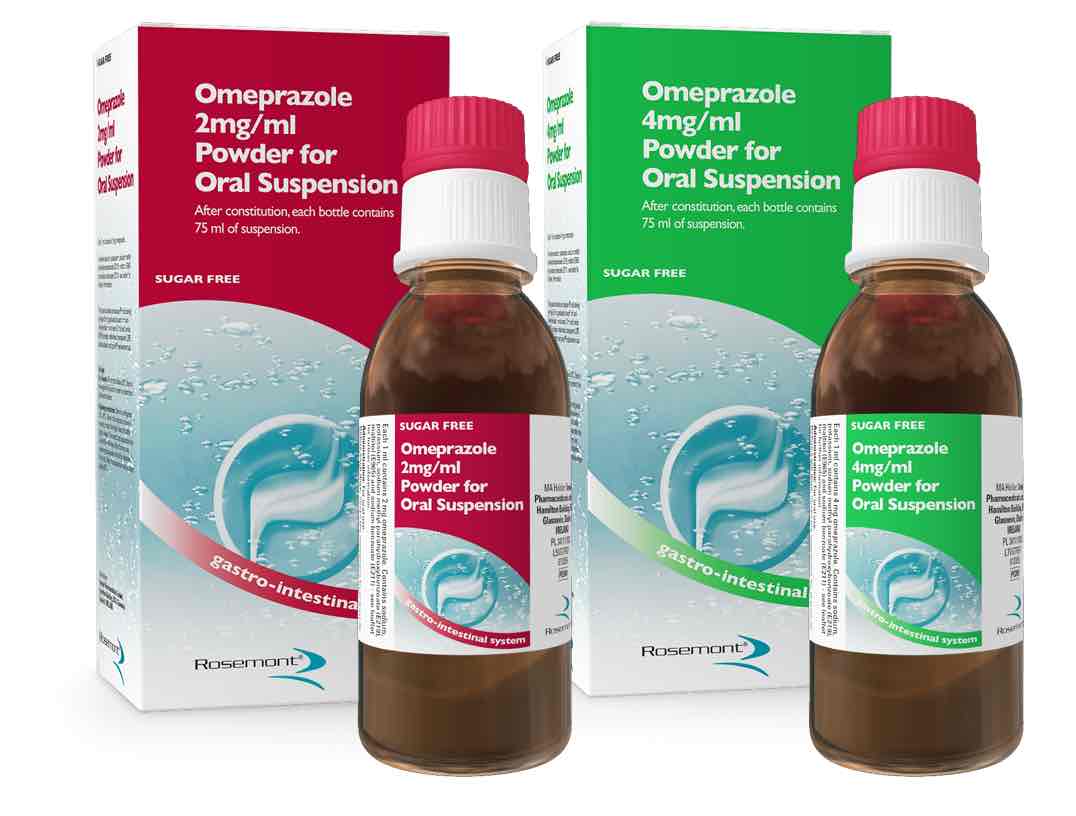
Rosemont Pharmaceuticals has launched omeprazole powder for oral suspension, the first PPI approved for use on a mg/kg basis, indicated in babies from one month and licensed for use with percutaneous endoscopic gastonomy (PEG) and nasograstic (NG) tubes. Available in 2mg/ml and 4mg/ml strengths, a patented pack makes constitution easy, says Rosemont. The 2mg strength has a natural vanilla flavour to improve palatability.
Further details are available from www.rosemontpharma.com
